
E-Medicare Pharma
Online Medicine Pharmacy
0.00$
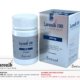
Azarest, containing Azacitidine as the active ingredient, is prescribed to adult patients diagnosed with intermediate-2 and high-risk myelodysplastic syndromes (MDS), including chronic myelomonocytic leukemia (CMML). Azacitidine acts as a demethylating agent by incorporating into DNA and RNA, leading to DNA hypomethylation and direct cytotoxic effects on abnormal hematopoietic cells. The recommended dosage of Azarest is 300 mg administered subcutaneously or intravenously once daily for 7 days, followed by a 21-day rest period, with treatment cycles repeated based on patient response. Common side effects include gastrointestinal symptoms, fatigue, and hematologic abnormalities, while serious adverse reactions may include bone marrow suppression and infections. Close monitoring for adverse effects and appropriate supportive care are essential in case of overdose, as there is no specific antidote available.
Interactions: Azarest may interact with drugs excreted renally or affecting renal function. Avoid co-administration with nephrotoxic agents and use caution with drugs causing myelosuppression or impacting hepatic function.
Precautions and Warnings:
Overdose Effect: In the event of overdose, provide supportive care. There’s no specific antidote; monitor for signs of myelosuppression and other adverse reactions, initiating appropriate symptomatic treatment as necessary.
| Product Name | Azarest |
|---|---|
| Generic Name | Azacitidine INN |
| Formulation | Tablet |
| Available Pack Size | 14 Tablets |
| Available Strength | 300 mg |

Previous Product
Next Product
Begin your journey to better health . Join now to unlock exclusive tips and insights sent straight to your inbox, empowering you to embrace a healthier lifestyle each and every day.

Online Medicine Pharmacy
+8801929123476 emedicarepharma@gmail.com
65, mymansingh High-way, Dhaka-1230, Bangladesh